Actis accelerate.
An incredibly fast way for medical devices to enter a market.
Actis Accelerate provides a unique pathway that many aren’t aware of.
It allows companies to fast-track the commercialization of their medical devices
by utilizing Actis’ existing ISO 13485, MDSAP, and MDR regulatory approvals, simplifying
market entry and cutting down on the time and cost of independent certifications.
a quick overview
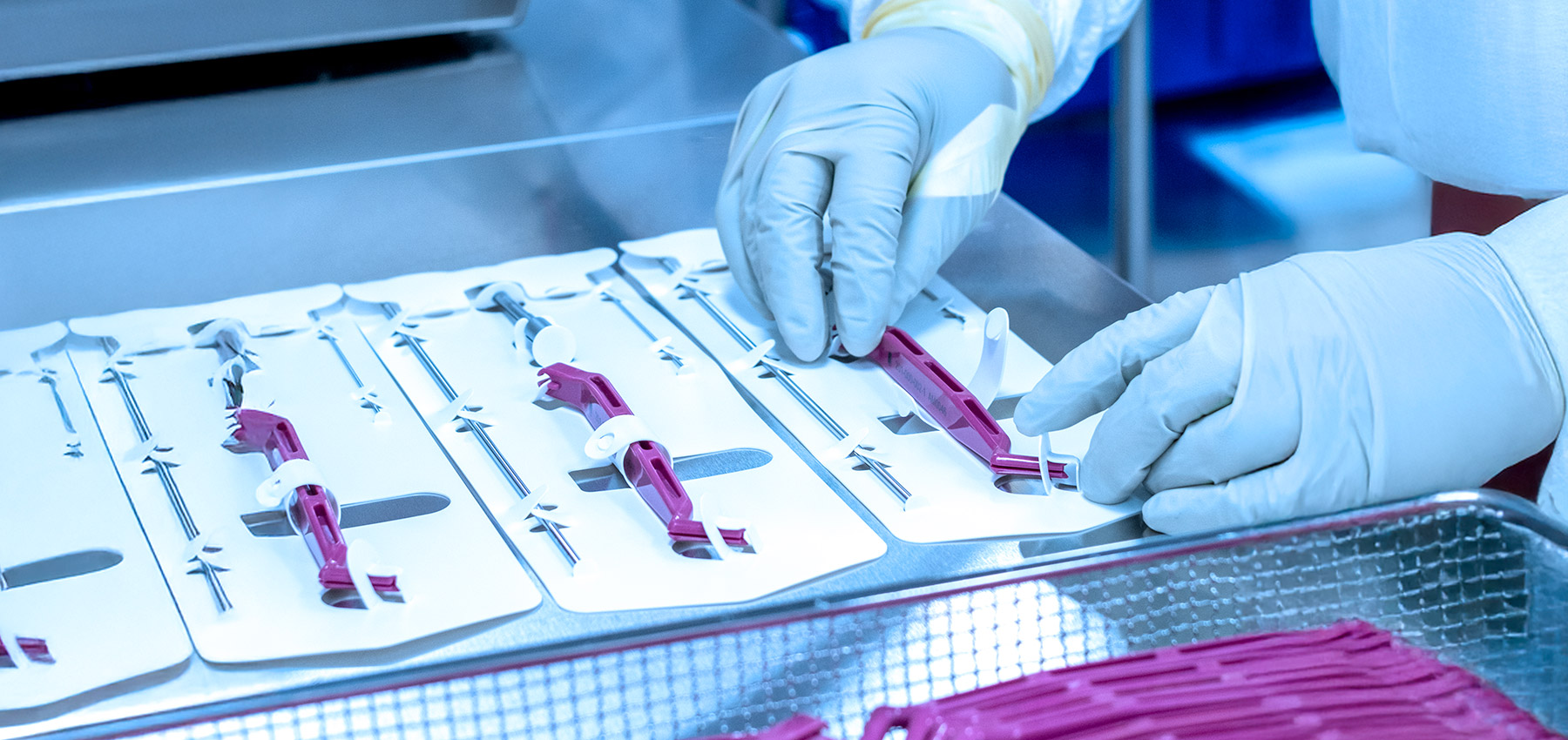
- The Actis manufacturing facility is proudly ISO 13485, MDSAP and MDR certified.
- Our certifications combined are recognised in over 40 different countries.
- Medical Device Single Audit Program. (MDSAP) allows auditing in a single program that satisfies the requirements of the participating regulatory authorities including the TGA, FDA, Brazil, Canada and Japan.
- The TGA recognises MDSAP certificates to support faster, more efficient medical device approvals — a powerful advantage for companies entering the Australian market.
- MDSAP is increasingly adopted by medical device companies seeking a streamlined, globally accepted route to meet regulatory expectations in Australia, the US, Canada, Japan and Brazil.
- MDSAP enables consolidation of global regulatory assessment processes across different countries. This reduces costs and increases predictability across different regions.
common questions
A wide range of medical devices can leverage the Actis Accelerate pathway, including implants, sterile consumables or kits, and surgical instruments. Actis Medical has particular expertise in orthopedic devices and patient matched medical devices, ensuring that even complex, high-precision technologies can be efficiently brought to market.
With Actis Accelerate, companies can access over 40 countries, including major markets such as Australia, the US, Europe, and Canada. This global reach ensures faster entry into highly regulated healthcare markets without the typical delays of independent regulatory approval.
It’s highly beneficial for Actis to be involved from the start of the process. We’ve encountered situations where clients attempted to handle it on their own, only to find the requirements too complex and time-consuming. Actis can develop a framework for your product and strategize the most effective way to launch it to market.
Yes—Actis Accelerate can reduce time to market and product launch by up to 50%**. You retain full IP ownership and product control, while leveraging Actis’ certifications and expertise to fast-track entry into global markets.
A wide range of medical devices can leverage the Actis Accelerate pathway, including implants, sterile consumables, and surgical instruments. Actis Medical has particular expertise in orthopedic devices and patient matched medical devices (PMMD’s), ensuring that even complex, high-precision technologies can be efficiently brought to market.
“Our start-up company faced immense challenges bringing our surgical device to market. We had invested time and money into consultancy with little result. We thought our only option was to establish an in-house regulatory department until an industry leader suggested we speak to the team at Actis Medical.
Since then, the “hands on” Actis Medical approach has seen the commercialisation of our device move quickly and efficiently. They offer a range of services unique to the market and we thoroughly recommend working with them.”
— Ben Littlejohns, Managing Director, JB Surgical.
